Memorial Sloan Kettering Library
COVID Impacts: Immune Dysfunction
Szczegółowe informacje i zasoby na temat długoterminowych konsekwencji zdrowotnych zakażenia COVID-19 oraz szerokich skutków społecznych pandemii COVID-19.
One of the most concerning long-term impacts of COVID-19 is immune dysregulation and dysfunction. Immune system impacts were heavily documented, even in the first waves of the pandemic, however there was a lack of understanding as to what exactly COVID-19 infections were doing to the immune system, and what that might mean both during acute infection and long-term.
Early Hypotheses
Early on in the pandemic, there were two main hypotheses for the pathophysiology of COVID-19 severe disease and death: hyperactive immune system and immune system failure.
Hyperactive Immune System
The first was due to an overactive immune system. Early on it was noted that many patients with severe COVID-19 ended up developing ARDS (acute respiratory distress syndrome). This was reminiscent of the cytokine release syndrome (CRS) - induced ARDS and secondary hemophagocytic lymphohistiocytosis (sHLH) that had been observed previously in patients with SARS-CoV and MERS-CoV (it also is a common adverse event in cancer patients treated with CAR-T cell therapies).
Therefore it lead researchers to believe that severe infections were the results of an overactive immune response caused by excessive inflammatory cytokines, which lead to inflammatory lung and vascular injuries, and that death was from subsequent respiratory failure or coagulopathy.
Immune System Failure
The second hypothesis took the exact opposite hypothesis, that COVID-19 caused immune collapse. In this hypothesis, COVID-19 causes the patient's protective immunity to collapse, causing uncontrolled viral replication and dissemination which lead to cytotoxicity and death. Support for this contrasting theory was based on the observed progressive and profound lymphopenia, often to levels seen in patients with AIDS.
More recent research has concluded that COVID-19 causes dysregulation to both the innate and the adaptive immune systems. Paradoxically, in COVID-19 pneumonia, the innate immune system fails to mount an effective antiviral response while also inducing potentially damaging inflammation.
COVID-19 Alters Both Innate and Adaptive Immunity
The immune system is made up of two parts: the innate, (general) immune system and the adaptive (specialized) immune system. These two systems work closely together and take on different tasks.
Innate Immunity
Responsible for the initial immune response and antiviral activity, the innate system functions as a single defense mechanism, crucial for host response and illness protection.
Severe COVID cases were found to have decreased production of early immune responses (INF) which in turn lead to the virus replicating and causing severe cellular lung damage. Not only is was the antiviral response of IFN delayed and reduced, but it was also accompanied an overexaggerated inflammatory response with excessive cytokines. This resulting hyperinflammation caused edema, fibrosis, and thromboses in the lungs that ultimately lead to hypoxia, acute respiratory distress syndrome (ARDS) and death.
Adaptive Immunity
The adaptive immune system is critical for the development of efficient host responses to invading pathogens as well as immunological memory for future infections of similar pathogens.
Although COVID-19 patients may exhibit elevated levels of inflammatory cytokines compared to non-critically-ill patients, a study comparing the immune profiles of COVID-19 and influenza noted that while a 3–4% subset of COVID-19 patients exhibited hyperinflammation characteristic of a cytokine storm, they more commonly demonstrated immunosuppression.
CD4+ helper T cells and CD8+ cytotoxic T cells have been identified as crucial in the immunologic response to SARS-CoV-2 infection. CD4+ T cells are responsive to the virus's spike protein, and the presence of CD8+ T cell expansion in bronchoalveolar lavage is correlated with illness moderation. However, one of the most remarkable characteristics of immune dysregulation in COVID-19 is an immense depletion of CD4+ and CD8+ T cells associated with disease severity.
While lymphopenia is observed in other respiratory viral illnesses such as influenza A H3N2 viral infection, COVID-19 induced lymphocytic depletion is distinctive for its magnitude and longevity. Additionally, CD8+ T cells, crucial for their cytotoxic activity against virally infected cells, may experience the more stark reduction.
The lack of intense lymphocytic infiltration found in the lungs of critical COVID-19 patients demonstrates that the peripherally observed lymphopenia may be occurring through a mechanism beyond simply recruitment to the infection site.
- Mohandas S, Jagannathan P, Henrich TJ, et al. Immune mechanisms underlying COVID-19 pathology and post-acute sequelae of SARS-CoV-2 infection (PASC). Elife. 2023;12:e86014. Published 2023 May 26.
- Klein J, Wood J, Jaycox JR, et al. Distinguishing features of long COVID identified through immune profiling. Nature. 2023;623(7985):139-148.


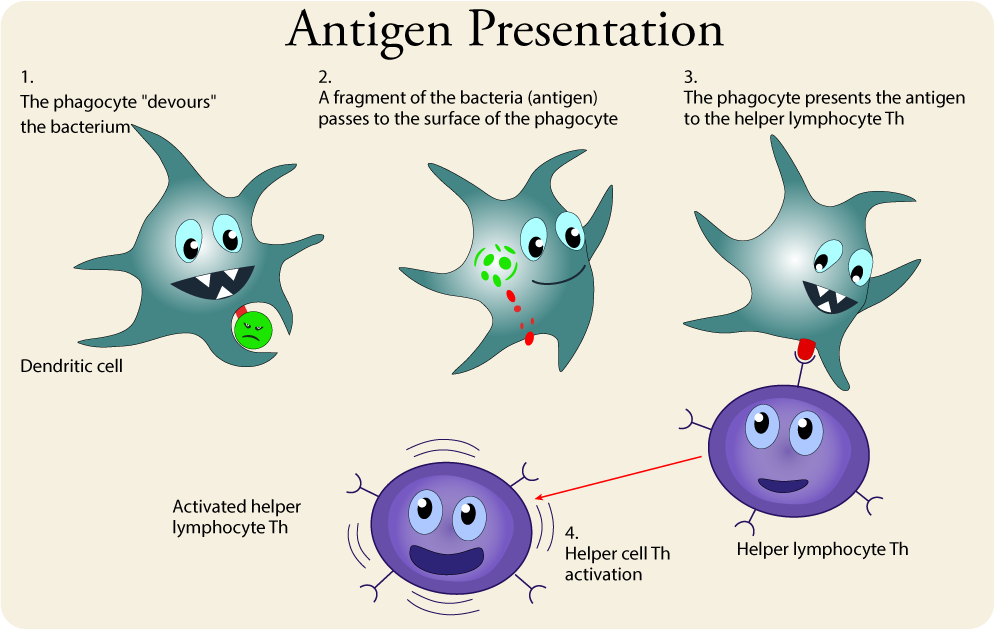
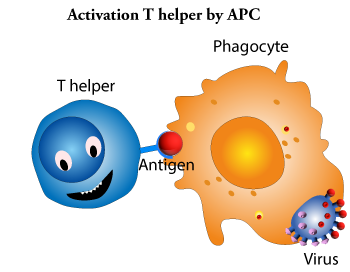 Th helper lymphocytes are the main driving force and regulator of the immune system. Their primary task is to activate B lymphocytes and killer T lymphocytes. However, the Th helper cells themselves must be activated first. This happens when a macrophage or dendritic cell that has previously absorbed the intruder moves to a nearby lymph node and presents information about the caught pathogen. The phagocyte presents a fragment of the intruder's antigen on its surface in a process known as antigen presentation. A Th helper cell is activated when its receptor recognizes an antigen. Once activated, the Th helper cell begins to divide and produce proteins that activate B and T cells as well as other cells of the immune system.
Th helper lymphocytes are the main driving force and regulator of the immune system. Their primary task is to activate B lymphocytes and killer T lymphocytes. However, the Th helper cells themselves must be activated first. This happens when a macrophage or dendritic cell that has previously absorbed the intruder moves to a nearby lymph node and presents information about the caught pathogen. The phagocyte presents a fragment of the intruder's antigen on its surface in a process known as antigen presentation. A Th helper cell is activated when its receptor recognizes an antigen. Once activated, the Th helper cell begins to divide and produce proteins that activate B and T cells as well as other cells of the immune system.